
Exploring Medical Research
– Zora Neale Hurston
Whole body donation plays a huge role in medical research and education. Cadavers offer a type of hands-on experience that cannot be recreated through technology and are used by medical students, physicians, researchers, and scientists to study things such as anatomy, identify disease sites, or determine causes of death. Typically, medical research can be broken up into three main categories.
- Basic Research
- Clinical Research
- Epidemiological Research
In medical research, there are two classified types – primary and secondary research. Secondary research is a summary of available studies in the form of reviews and analysis. Primary research is where the actual studies happen. Basic, Clinical, and Epidemiological research are classified as three types of primary research.
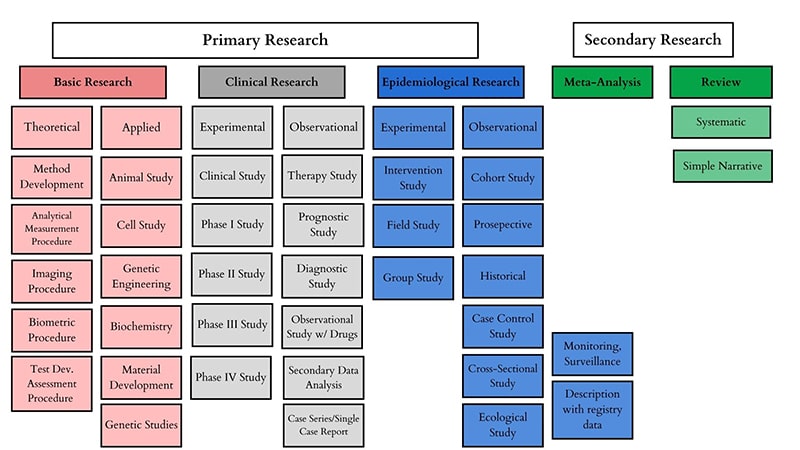
Basic Research
Basic research is technically fundamental research or the study of life processes that are universal in their application to scientific knowledge. David Frank, MD, Associate of Medicine, Medical Oncology at the Dana Farber Cancer Institute quoted basic research as:
“If you think of it in terms of construction, you can’t put up a beautiful, elegant house without first putting in a foundation. In science, if you don’t first understand the basic research, then you can’t move on to advanced applications.”
Basic medical research includes animal experiments, cell studies, biochemical, genetic, physiological investigations, and studies on the properties of drugs and materials in fields such as biology and chemistry. These studies are the study of the core building blocks of life including DNA, cells, proteins, and molecules to help answer the questions about their structures and how they work.
Typically, in these experiments there is at least one independent variable that is varied, and those effects are what are investigated. The variable could be the population, number of groups, case numbers, treatments, or dosages. Also in these experiments, the confounding factors should be controlled or reduced, specific hypotheses are investigated, and causal statements are made.
Basic research will also include the development and improvement of analytics procedures. These procedures can include but are not limited to:
Analytical determination of
- Enzymes, markers, or genes
Imaging procedures
- Computed tomography
- Magnetic resonance imaging
Gene Sequencing
Development of Biometric procedures
- Statistical test procedures
- Modeling and statistical evaluation strategies
Clinical Research
Clinical research is a step above basic and addresses important questions of normal function and disease by using human subjects. This type of research is geared more toward exploring whether new treatments, medications and diagnostics techniques are not only safe but effective as well.
Clinical trials are extremely rigorous and controlled so that they can accurately monitor progress and evaluate the treatment’s efficacy and benefits. Clinical research can be broken down into several distinct categories of study. Some of those include but are not limited to:
Treatment Research that involves the intervention of medicine, psychotherapy, new devices and innovative approaches to surgery or therapy.
Prevention Research which aims to better the prevention of disorders from developing or returning using medicine, vitamins, vaccines, minerals, or lifestyle changes.

Diagnostic Research is the practice of looking for better ways to identify a particular disorder or condition.
Screening Research aims to find the best ways to detect certain disorders or health conditions.
Quality of Life Research finds new ways to improve comfort and quality of life for patients with chronic illnesses.
Genetic Studies are implemented to improve the prediction of disorders by identifying and understanding how genes and illnesses may be related.
Research includes discovering why a person’s genes make them more or less likely to develop certain disorders.
While clinical research is commonly associated with new medications or devices, some studies include healthy volunteers that are needed to compare to results of patients with illnesses. These types of studies include:
- Long-term studies that involve psychological tests or brain scans
- Genetic studies that involve blood tests
- Studies of family history
Epidemiological Research
This specific branch of medical research investigated all the factors that determine the presence or absence of disease and disorders to help understand how many people have a disease or disorder, if those numbers are fluctuating and how it affects our society and economy.
With epidemiology, the patient is the community and individuals that are viewed collectively. Epidemiology is the study (scientific, systematic and data drive) of the distribution (pattern and frequency) and determinants (causes and risk factors) of health related states and events in specific populations (neighborhoods, schools, states, cities, global).
When it comes to epidemiological studies there are some key terms you need to know:
1. Incidence: number of new cases in the population over a set period
2. Prevalence: number of exciting cases
3. Cost of illness: expenditures of medical care
4. Burden of disease: total significance of disease for society
- Measured in years of life lost to ill health
- Difference in total life expectancy and disability-adjusted life expectancy (DALY)
5. DALY – summary measure of health of a population. One DALY is one lost year of health and is used to estimate the gap between the current health of the world and an ideal situation.
There are several health problems or events that Epidemiological researchers are studying. These include environmental exposures like lead, heavy metals, air pollutants or asthma triggers. Infectious diseases such as foodborne illness, influenza, pneumonia. Injuries including increased homicides in a specific community or a national surge of DV. Non-infectious diseases that can include localized or widespread rise of a particular type of cancer or an increase in a major birth defect.



