Medical Implants
Medical implants are man-made devices that are meant to replace missing biological structures, support damaged biological structures, have therapeutic purposes, collect diagnostics, and help maintain daily body functions.
Implants can be used within the human body or on the surface of the body either temporarily or permanently. There is a vast number of implants that aid with sensory issues, neurological disorders, cardiovascular disorders, contraception, pain-relief, alleviation of bone or joint issues, and a variety of organ dysfunctions.
So why are implants a game-changer? A huge reason is because medical implants are equipped with unique responsive polymers that are used to facilitate deployment and ensure the removal of devices with minimal damage to tissue. These unique polymers support important functions such as treating ailments, delivering drugs, controlling infections and monitoring physiological factors. Typically, the application of these polymers is divided by application areas that include:
- Cardiovascular Devices
- Respiratory Devices
- Surgical Devices
- Dental Devices
- Orthopedic Devices
- Ophthalmic Devices
- Gastrointestinal Devices
- Drug Delivery Devices
- Implantable Biosensors
- Urogenital Devices
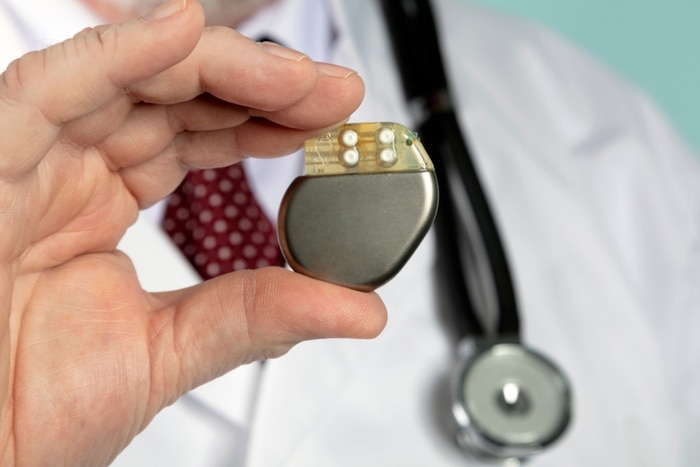
Since the first pacemaker implant in 1958 there has been a drastic advancement in all areas such as battery power, functionality, power consumption, and system delivery. One of the most beneficial advancements in the improvement of surgical implant devices and how they operate has to do with whole body donation. This is because body donation allows surgeons and medical professionals hands-on access to cadavers where they are given the opportunity to not only perfect surgical techniques but get an early introduction to new devices. This gives medical professionals a firsthand look with implants as they are created and introduced into the industry.
Another huge step forward for surgical implants was introducing different types of material that are tailor made for different necessities that help reach desired results. As implants improve, materials have become more lightweight, hypoallergenic, and biocompatible. Introducing new materials help ensure desired results with minimum discomfort, or risk of second surgeries, for patients. Current common materials for implants are:
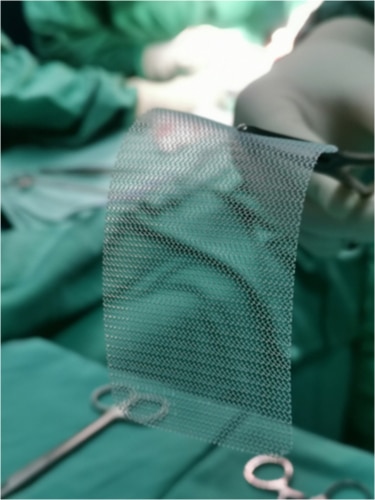
Surgical Mesh – made from inorganic or biological materials that are woven to form a sheet. Surgical mesh can be used permanently or temporary to help support organs or other tissues.
Polyethylene – a common plastic that is often used for medical implants because it does not degrade in the body. Polyethylene is typically used for knee or hip replacement implants.
Titanium – a light, extremely strong, non-allergenic and biocompatible metal that is often used to make implants for dental surgeries. Lately, it has also been used for other medical uses such as hip implants, heart valves and bone screws.
Polyurethane Foam – a fairly new addition to surgical implant materials, this foam is created by combining memory polyurethane foam with the bone tissue mineral “hydroxyapatite” and specifically encourages the regeneration of bone. They are most common in short term implants such as catheter tubing, wound dressing and injection molded devices.
Polylactic Acid – Often patients with titanium screws need to have a second operation to get them removed to combat this, surgeons opt for polylactic screws due to them being biocompatible and biodegradable.
3-D Printed Biomaterials – this technology uses a microfluidic approach and a device filled with stem cell “ink” that allows repair of damaged bone and cartilage offering precise replications of human tissue.
As advancements are made, the variety of available implants continues to grow rapidly. In just 2021 alone, there have been an impressive forty-one medical devices cleared and approved by the FDA that range from stent systems, standard implants, catheters, and gel implants. Annually some of the most common implantable devices remain to be:
1. Implantable Cardioverter Defibrillators
a. 800,000 people currently have ICDs
b. 100,000 are implanted annually
2. Artificial Hips
a. 2.5 million currently have artificial hips
b. 450,000 hip replacements are performed annually
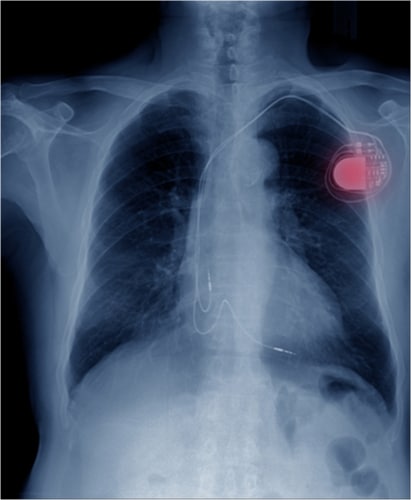
3. Heart Pacemakers
a. Three million people currently have pacemakers
b. 200,000 pacemakers are implanted annually
4. Spine Screws, Rods and Artificial Disks
a. 500,00 spinal fusion surgeries are performed annually
5. Artificial Knees
a. 4.7 million people had knee replacements
b. Almost one million procedures are performed annually
6. Coronary Stents
a. More than eight million people currently have stents
b. Two million stents are inserted yearly
7. Ear Tubes
a. One in fifteen kids get ear tubes before age three
b. 500,000 ear tubes are implanted annually
8. Artificial Eye Lenses
a. Six million lenses are implanted annually
Medical implants are a thriving sector of medical technology with new implants emerging regularly in the market. It is currently dominating the market at an impressive $89 million in 2021. With versatility and innovation directly meeting patient needs, implants are proving to be a safe, effective, and prevalent option for a variety of conditions and patients.